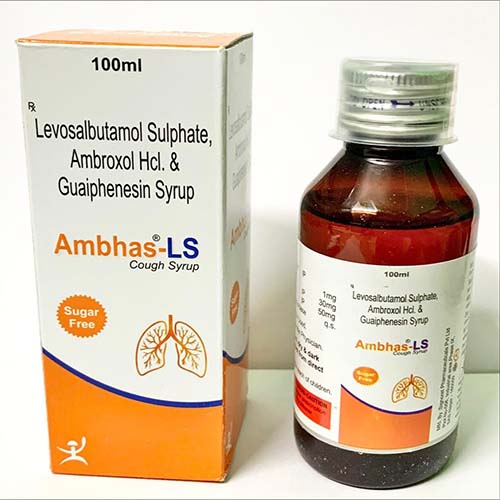
Ambhas LS Syrup is a combination medicine is used to relieve symptoms such as wheezing, shortness of breath, chest tightness, breathing difficulties, coughing, etc. caused by bronchospasm in conditions such as bronchial asthma, chronic bronchitis, chronic obstructive pulmonary disease (COPD), etc.
Uses of Ambhas LS Syrup
What is it prescribed for?
Bronchospasm
This medicine is used to relieve the symptoms of bronchospasm such as wheezing, shortness of breath, chest tightness, breathing difficulties, coughing, etc. in patients suffering from conditions such as bronchial asthma, chronic bronchitis, etc.
How long does it take for this medicine to take effect?
The effect of this medicine can be observed within 5-30 minutes of administration of the dose.
When not to use?
Allergy
This medicine is not recommended for use in patients with a known allergy to levosalbutamol, salbutamol, guaifenesin, ambroxol, or any other inactive ingredients present along with these medicines.
Gastric ulceration
This medicine is not recommended for use in patients with a known history of gastric ulceration due to the increased risk of worsening of the patient’s condition.
Stevens-Johnson syndrome
This medicine is not recommended for use in patients with a known history of Stevens-Johnson syndrome which is a rare but serious disorder of the skin due to the increased risk of worsening of the patient’s condition.
Warnings
Warnings for special population
Pregnancy
This medicine is not recommended for use in pregnant women unless necessary. All the risks and benefits should be discussed with the doctor before taking this medicine.
Breast-feeding
This medicine is not recommended for use in breastfeeding women unless necessary. All the risks and benefits should be discussed with the doctor before taking this medicine. If the medicine is used, the infant should be monitored closely for any undesired side effects.
General warnings
Severe kidney function impairment
This medicine should be used with caution in patients suffering from severe kidney function impairment due to the increased risk of worsening of the patient’s condition. Close monitoring of kidney function, appropriate dose adjustments, or replacement with a suitable alternative may be necessary based on the clinical condition of the patient.
Severe liver function impairment
This medicine should be used with caution in patients suffering from severe liver function impairment due to the increased risk of worsening of the patient’s condition. Close monitoring of liver function, appropriate dose adjustments, or replacement with a suitable alternative may be necessary based on the clinical condition of the patient.
Use in Children
This medicine should be used with extreme caution in children and only under the supervision of a qualified healthcare professional.
Cough suppressants
This medicine is not recommended for use in patients taking cough suppressants since they have opposite effects. Coadministration of these medicines may result in severe adverse effects and worsen the patient’s condition.
Driving or Operating machinery
This medicine may cause symptoms such as dizziness, a shakiness of arms and legs, muscle weakness, etc. in some patients. It is advised that you do not perform any activities such as driving a vehicle or operating machinery if you experience any of these symptoms during treatment with this medicine.
Other medicines
This medicine may interact with many other medicines and may cause severe adverse effects. Hence, it is advised that you inform the doctor about all your current medicines including any herbs and supplements before beginning treatment with this medicine.
Other diseases
Report the past/present incidence of all diseases including heart and blood vessels disorders, electrolyte imbalance, any disease associated with the thyroid and/or other glands, etc., to the doctor before beginning treatment with this medicine.
Use in elderly
This medicine should be used with caution in the elderly patients since the risk of adverse effects is significantly high. Appropriate dose adjustments may be necessary based on the clinical condition of the patient.
How long do the effects of this medicine last?
The amount of time for which this medicine remains active in the body is not clinically established.
Is it safe to consume alcohol while taking this medicine?
Interaction with alcohol is unknown. It is advisable to consult your doctor before consumption.
Is this a habit forming medicine?
No habit-forming tendencies were reported.


Reviews
There are no reviews yet.